Centaur Clinical CRO
Centaur Clinical, your expert CRO in clinical research for pharmaceuticals, medical devices, and IVDs





Welcome to Centaur Clinical CRO,
your expert partner in clinical research.
Our strengths
Full clinical trial support: studies, CE marking, PMCF, and bioequivalence
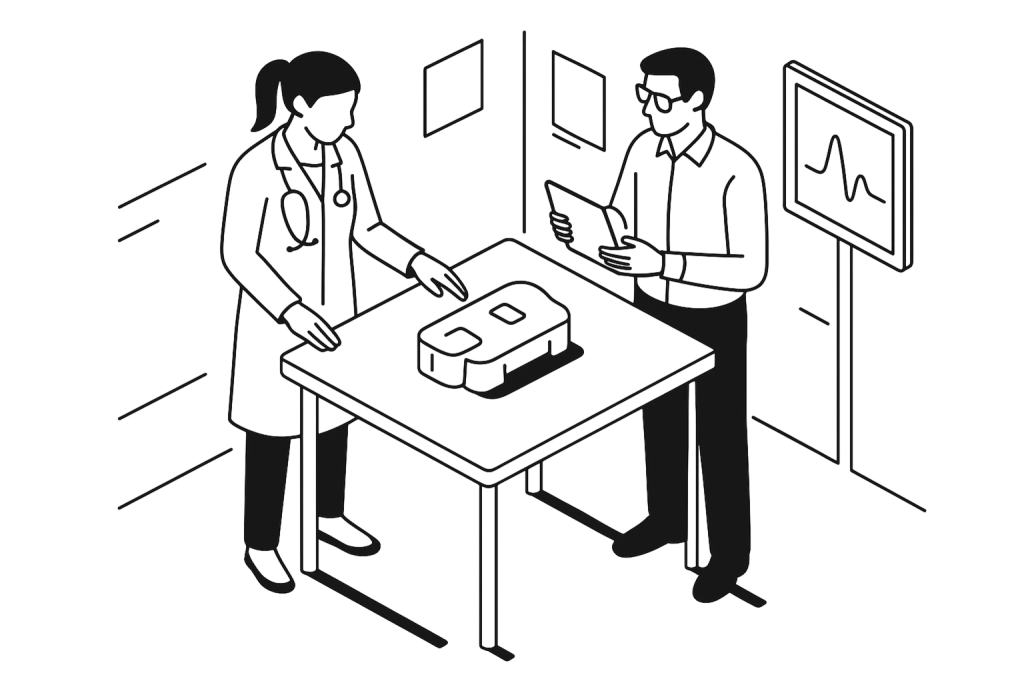
Centaur Clinical is a Contract Research Organization (CRO) specialized in clinical research. We support manufacturers of pharmaceuticals, medical devices (MD/IVD), dietary supplements and other healthcare products requiring clinical studies through an integrated service offering across our two sites.
CRO France: we support manufacturers throughout the entire lifecycle of their clinical operations, including CE marking activities and Post-Market Clinical Follow-up (PMCF).
CRO Algeria: we manage the conduct of clinical studies and investigations, including clinical trials and bioequivalence studies.
We ensure scientific rigor, optimal regulatory compliance, and operational excellence throughout your projects.
Our services
Centaur Clinical, expert CRO in clinical research: comprehensive services to secure and manage your trials
Regulatory strategy – Planning – Medical writing – Study design – Implementation – Post-market surveillance – Data management – Bioequivalence
Data management
Centaur Clinical provides a dedicated Data Management service with a specialized team handling queries and CRF review. We also offer access to an eCRF compliant with FDA and European standards, at a competitive cost.
Clinical Trials
As a monitoring specialist, Centaur Clinical CRO manages your clinical trials from study design through post-market follow-up, in full compliance with Good Clinical Practice (GCP) and ISO 14155 standards.
Why trust us
Proven clinical expertise serving your medical devices and healthcare products
At Centaur Clinical, clinical research is driven by rigor, control, and excellence.
Expertise in clinical trials and patient safety monitoring
We provide pharmacovigilance and materiovigilance services to ensure patient safety, data integrity, and full compliance with international regulatory requirements throughout your clinical projects.
Multisector expertise, including rare diseases
We support a wide range of therapeutic areas, from common conditions to rare diseases.
Our teams design and conduct robust, compliant clinical trials aligned with international standards.
Versatility and operational excellence
Our versatility allows us to adapt to the specific requirements of each project while maintaining rigor, compliance, and operational excellence.
Guaranteed safety and compliance
We ensure enhanced safety for your medicines, medical devices, and in vitro diagnostics, supported by comprehensive CE marking expertise and proven mastery of regulatory processes.
Support at every stage of development
Through an integrated approach, we assist manufacturers of pharmaceuticals and medical devices from study design to clinical follow-up, ensuring:
- High-quality data
- Patient safety
- Efficient and controlled processes
Expert oversight and proactive project management
Our experienced teams ensure smooth coordination, proactive risk anticipation, and rigorous monitoring to secure the efficiency and success of your clinical projects.
Expertise in clinical trials and patient safety monitoring
We provide pharmacovigilance and materiovigilance services to ensure patient safety, data integrity, and full compliance with international regulatory requirements throughout your clinical projects.
Guaranteed safety and compliance
We ensure enhanced safety for your medicines, medical devices, and in vitro diagnostics, supported by comprehensive CE marking expertise and proven mastery of regulatory processes.
Multisector expertise, including rare diseases
We support a wide range of therapeutic areas, from common conditions to rare diseases.
Our teams design and conduct robust, compliant clinical trials aligned with international standards.
Support at every stage of development
Through an integrated approach, we assist manufacturers of pharmaceuticals and medical devices from study design to clinical follow-up, ensuring:
- High-quality data
- Patient safety
- Efficient and controlled processes
Versatility and operational excellence
Our versatility allows us to adapt to the specific requirements of each project while maintaining rigor, compliance, and operational excellence.
Expert oversight and proactive project management
Our experienced teams ensure smooth coordination, proactive risk anticipation, and rigorous monitoring to secure the efficiency and success of your clinical projects.
Clinical performance and operational excellence
A CRO committed to the success of your projects
Efficient and structured study management
We manage your clinical trials with rigor, foresight, and seamless coordination to ensure secure and controlled clinical development.
Clear communication and transparent reporting
You benefit from accurate project monitoring, regular exchanges, and full visibility on study progress at every stage.
Result-driven and compliance-focused methodology
Our approach is built on proven standards and best practices, ensuring operational efficiency, performance, and full regulatory alignment.
Tailored support aligned with your constraints
We adapt our actions to your strategic objectives, target markets, and operational priorities, delivering solutions that fit your specific challenges.
International presence with strong roots in France and Algeria
Based in Europe and Algeria, we support your clinical projects across Europe, the United States, the Middle East, and Africa.
Efficient and structured study management
We manage your clinical trials with rigor, foresight, and seamless coordination to ensure secure and controlled clinical development.
Clear communication and transparent reporting
You benefit from accurate project monitoring, regular exchanges, and full visibility on study progress at every stage.
Result-driven and compliance-focused methodology
Our approach is built on proven standards and best practices, ensuring operational efficiency, performance, and full regulatory alignment.
Tailored support aligned with your constraints
We adapt our actions to your strategic objectives, target markets, and operational priorities, delivering solutions that fit your specific challenges.
International presence with strong roots in France and Algeria
Based in Europe and Algeria, we support your clinical projects across Europe, the United States, the Middle East, and Africa.
I have a clinical project
Optimized clinical studies for your medical devices
Our team’s expertise enables us to design and conduct optimized clinical studies, regardless of the medical device class or therapeutic area.

Our clients
They trust us













News